
Chemistry Formula Sheet AP Chemistry Ion Sheet Chemistry Pinterest Ap chemistry
Writing word and symbol equations. Chemical equations can be written using either words or chemical symbols.With chemical equations, we often indicate the state symbols after each molecule or element in the symbol equations to indicate whether they are in solid, liquid, aqueous or gaseous form.For example, a displacement reaction between copper sulfate and magnesium, can be shown as follows:
HelpWork balancing chemistry equations
Chapter 11. Chemical Equations 11.1. ChemicalEquation Concepts Under the right circumstances substances change into newsubstances. Suchchanges are calledchemical transformations,orreactions.The original substances are calledreac- tants,the substances resulting from the change are calledproducts.Chemical reactions reveal the basic properties of substances that form the heart of chemistry.

List of chemical formula or common name of all compound Brainly.in Chemical
CHAPTER1 C onsider the following situations of daily life and think what happens when - milk is left at room temperature during summers. an iron tawa/pan/nail is left exposed to humid atmosphere. grapes get fermented. food is cooked. food gets digested in our body. we respire.
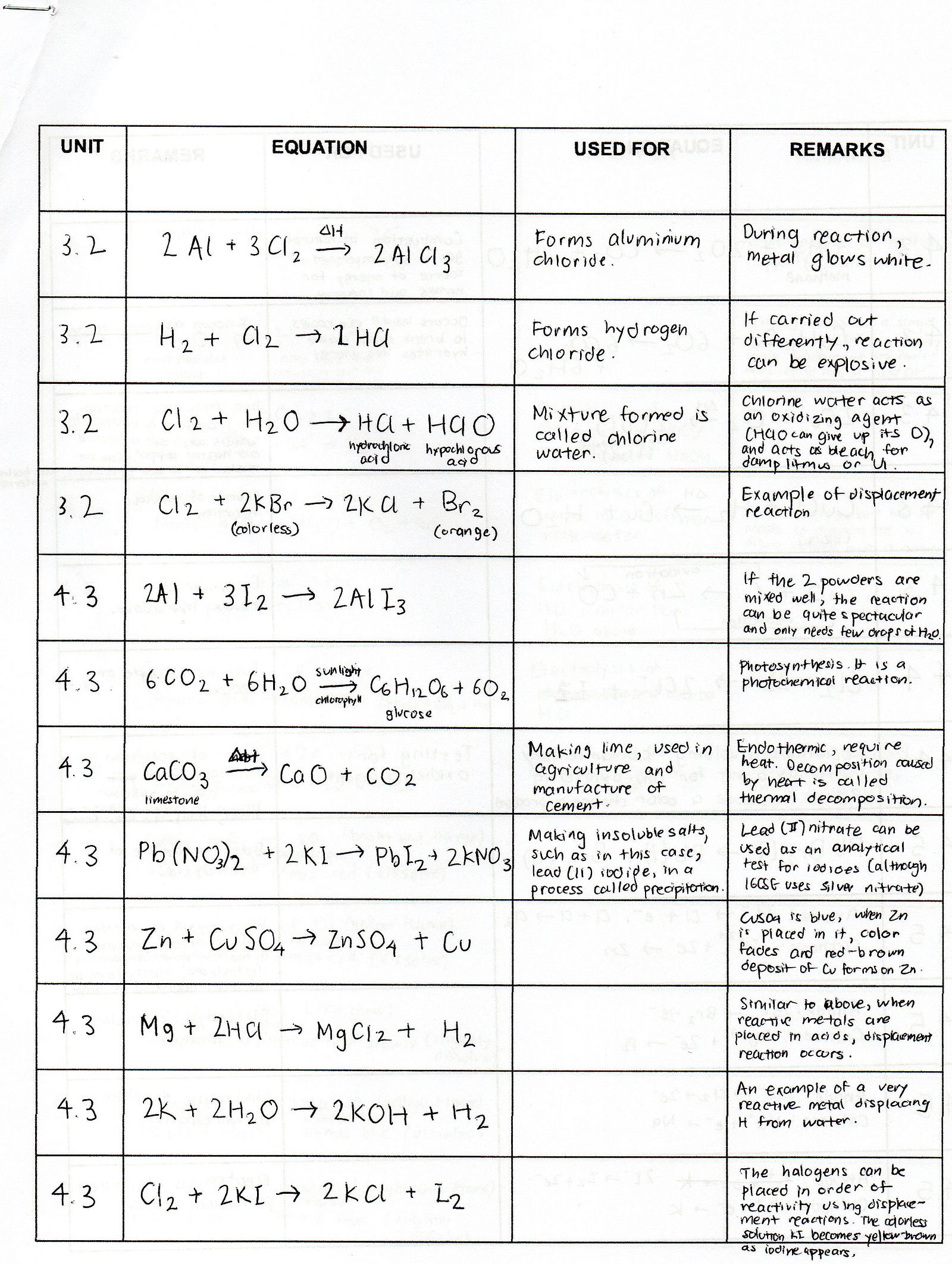
Chemistry Formulas And Equations Review Tessshebaylo
1 atom Fe 2 atoms O Right side: 2 atoms Fe 3 atoms O The balancing of the equation is accomplished by introducing the proper number or coefficient before each formula. To balance the number of O atoms, write a 3 in from of the O2 and a 2 in front of the Fe2O3: Fe + 3 O2 → 2 Fe2O3
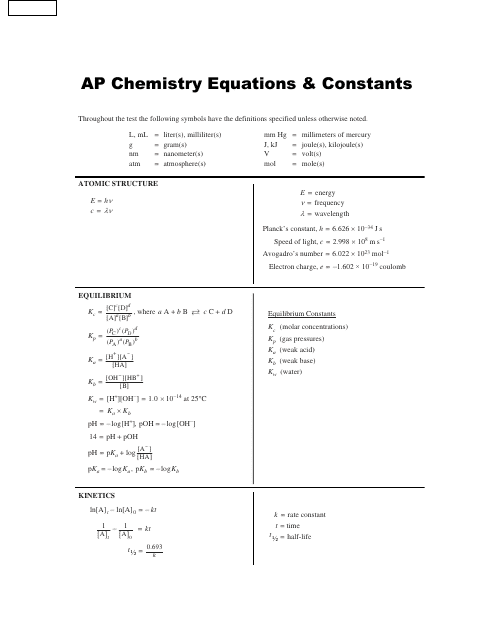
Ap Chemistry Equations and Constants Reference Sheet Download Printable PDF Templateroller
OBJECTIVES After completing this lesson, you will be able to: write and balance simple chemical equations; describe the significance of a balanced chemical equation; explore the relationship between mole, mass and volume of various reactants and products;
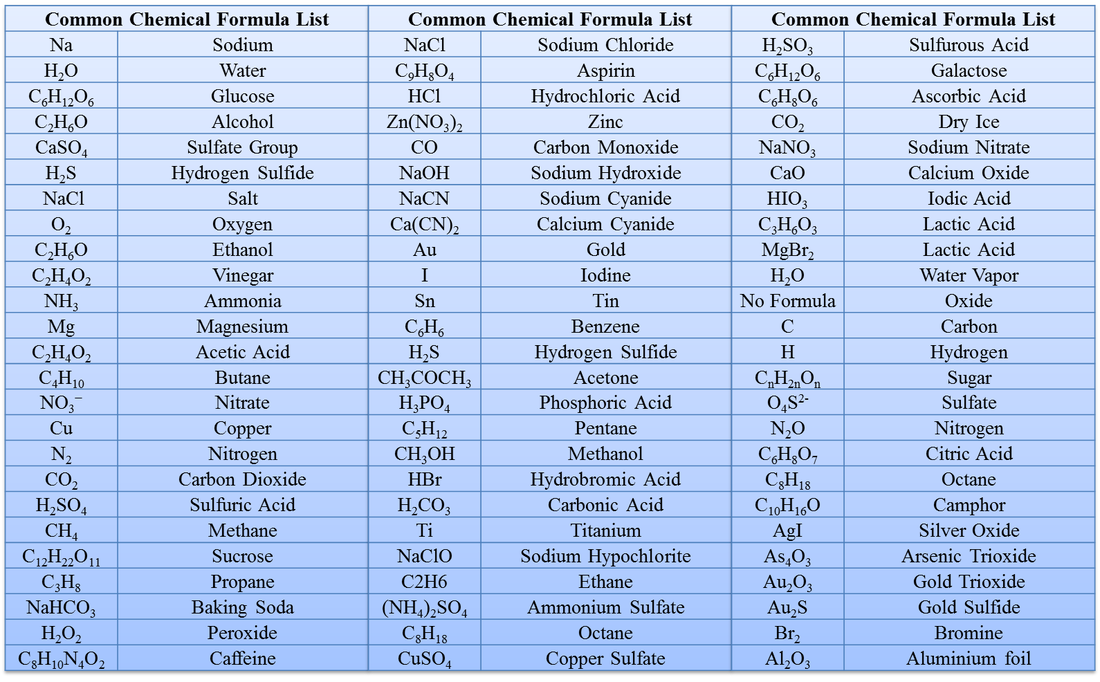
Chemical Formula ( रासायनिक नाम और सूत्र ) List, Table, Chart And PDF
A chemical equation describes what happens in a chemical reaction. The equation identifies the reactants (starting materials) and products (resulting substance), the formulas of the participants, the phases of the participants (solid, liquid, gas), and the amount of each substance.

Balancing Chem Equations WS1 PDF
Part of Chemistry (Single Science) The nature of substances and chemical reactions Remove from My Bitesize Balancing equations and calculations Word equations are useful to show which.

Balancing Chemical Equations Mr. Durdel's Chemistry WMS Chemistry classroom, Chemical
chemical reactions when the names of the reactants and products are specified; 1.5.8 write balanced ionic equations for reactions covered in this specification; 1.5.9 write half equations for reactions covered in this specification; 1.5.10 demonstrate knowledge and understanding that in chemical equations the three states of matter
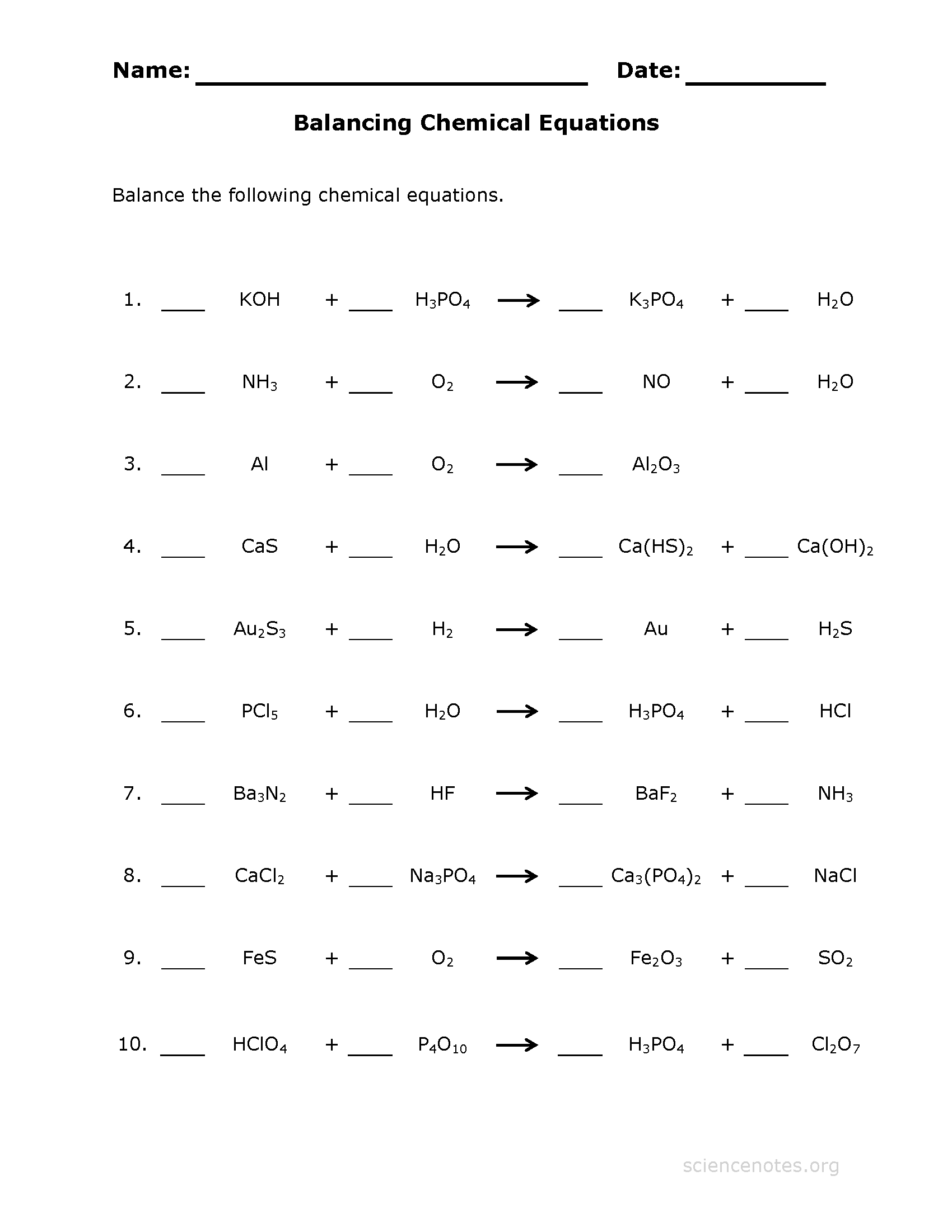
Balancing Chemical Equations Practice Worksheet —
CH4 +Cl2 → CCl4 + HCl (5.3.5) (5.3.5) CH 4 + Cl 2 → CCl 4 + HCl. Step 2: Count the number of each atom or polyatomic ion on both sides of the equation. Reactants 1Catom 4H ions 2Clatom Products 1Catom 1H ions 5Clatoms Reactants Products 1 C atom 1 C atom 4 H ions 1 H ions 2 Cl atom 5 Cl atoms.

Common Chemical Formula List Hydroxide Acid
The general equation for a chemical reaction is: As to why water is produced as a gas, it may be observed that the water is produced as a result of an explosion as shown in Figure 7.3.1 7.3. 1. At the elevated temperature of the explosion, water exists as a gas and not as a liquid.

Chemistry+Equation+Sheet.pdf Enthalpy Chemical Reactions
Types of Chemical Reactions Dissolution Precipitation Acids and Bases and their reactions Oxidation-Reduction Reactions Dissolution of Ionic Compounds Dissolution of Molecules Electrolytes Substances which increase the conductivity of water when they dissolve

WORKSHEET (chemical equations).pdf Chemical Reactions Unit Processes
A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a reaction. A chemical equation shows the starting compound (s)—the reactants —on the left and the final compound (s)—the products —on the right, separated by an arrow.
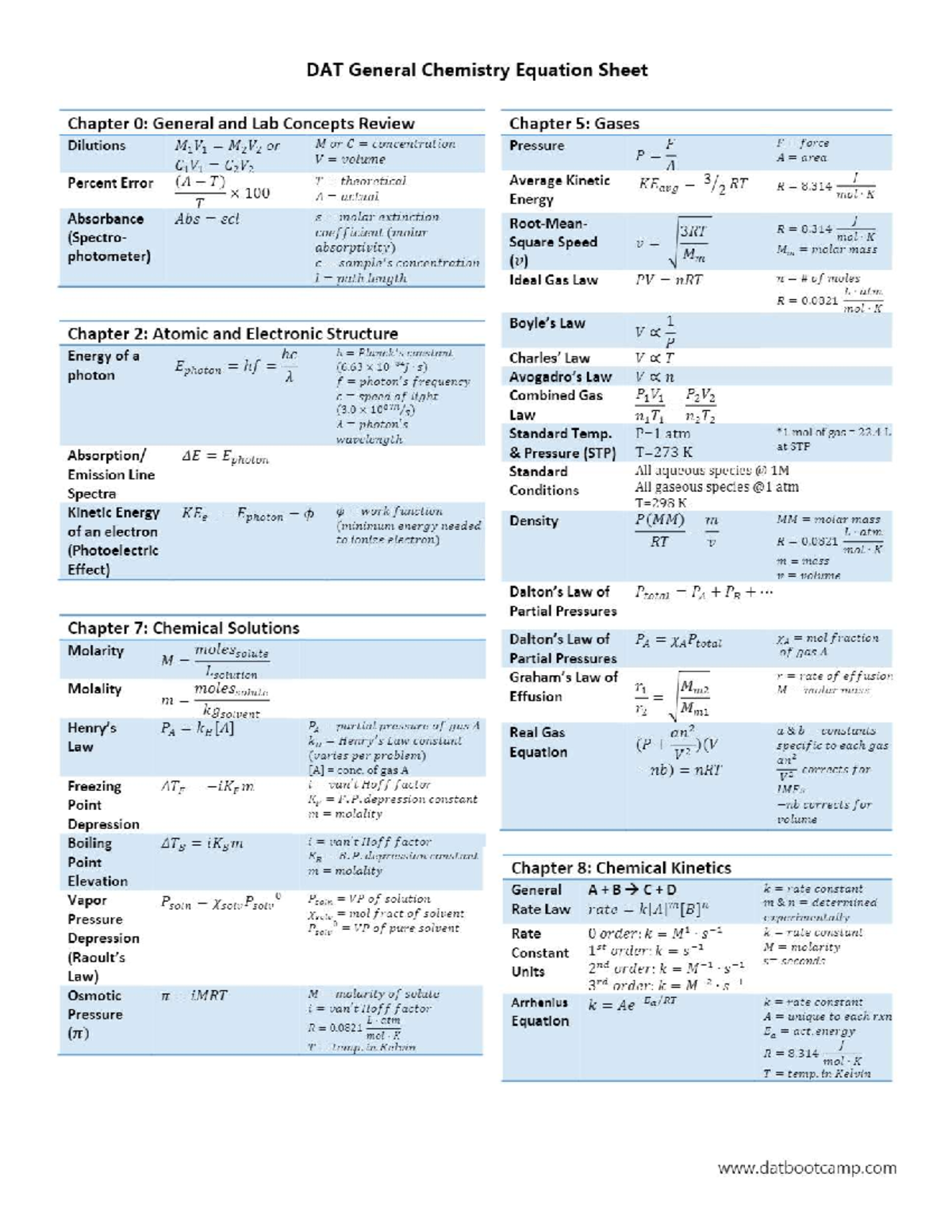
Chem equation sheet for chemistry class Eng Tech 1Ch3 McMaster StuDocu
Chemistry Basic Formulas & Equations Reference. The complete list of chemistry basic equations & formulas cheat sheet for PDF download to help users to use them offline to learn or workout how to execute or solve the various calculations of normality, molarity, molality, enthalpy, entropy, gas, energy, equilibrium and much more.

How to Write a Chemical Equation (with Pictures) wikiHow
To indicate more than one polyatomic ion in a chemical formula, place parentheses around the polyatomic ion and use a subscript. Roman numerals indicate the oxidation number of cations having multiple possible oxidation states. After reading Lesson 9.2, answer the following questions. Binary Ionic Compounds 1.
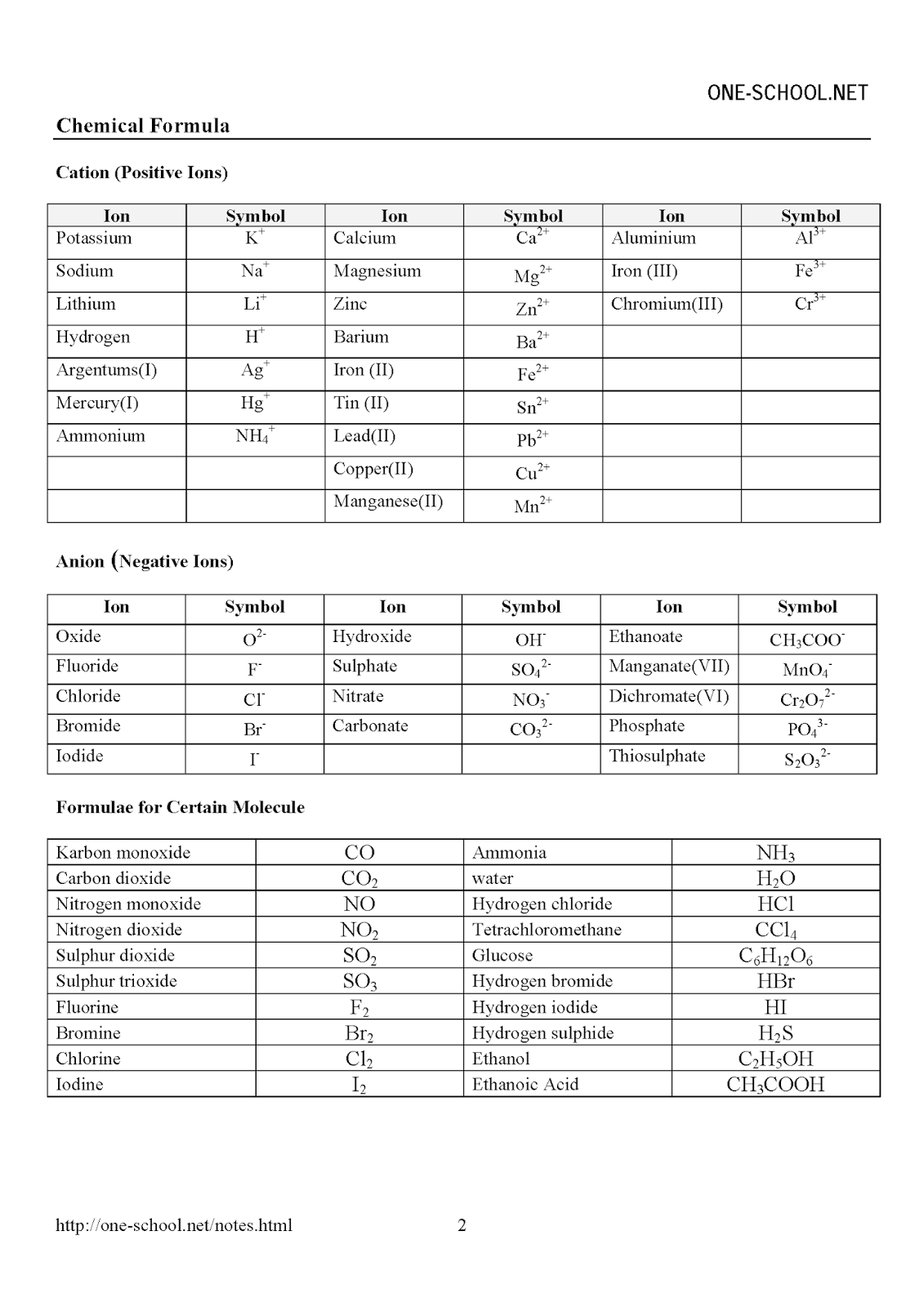
SPM Form 4 Chemistry Formulae List SPM Chemistry
Microsoft Word - Chapter 6.doc. 82. Returning to our earlier, mistake free equation: CH4 + O2. →. CO2 + H2O. We are ready for the second absolute requirement: "The number of atoms of each. element must be the same on both sides of the equation.". In our equation, we have.

Exemplary 100 Examples Of Chemical Equations Balanced Molecular Equation Calculator
The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter.